Title: Assessment of the Effects of Different Compositions of Ingredients Used on the Characteristics of an Emulsion Formulation
Objective: 1. To determine the effect of HLB surfactant on the emulsion stability. 2. To study the effect on physical and stability of the emulsion when the different amount of emulsifying agent have been used.
Introduction: An emulsion is a thermodynamically unstable two-phase system consisting of at least two immiscible liquids, one of which is dispersed in the form of small droplets throughout the other, and an emulsifying agent. The dispersed liquid is known as the internal or discontinuous phase, whereas the dispersion medium is known as the external or continuous phase. Where oils, petroleum hydrocarbons, and/or waxes are the dispersed phase, and water or an aqueous solution is the continuous phase, the system is called an oil-in-water (o/w) emulsion. Conversely, where water or aqueous solutions are dispersed in an oleaginous medium, the system is known as water-in-oil (w/o) emulsion. W/O emulsions tend to be immiscible in water, are occlusive, and may be “greasy.” This is primarily because oil is the external phase, and oil will repel any of the actions of water. The occlusiveness is because the oil will not allow water to evaporate from the surface of the skin. Conversely, o/w emulsions are miscible with water, are water washable, non-occlusive, and non-greasy. Emulsions are, by nature, physically unstable; that is, they tend to separate into two distinct phases or layers over time. Several levels of instability are described in the literature. Creaming occurs when dispersed oil droplets merge and rise to the top of an o/w emulsion or settle to the bottom in w/o emulsions. In both cases, the emulsion can be easily redispersed by shaking.
Coalescence (breaking or cracking) is the complete and irreversible separation and fusion of the dispersed phase. Finally, a phenomenon known as phase inversion or a change from w/o to o/w (or vice versa) may occur. Emulsions are stabilized by adding an emulsifier or emulsifying agents. The emulsifying agent can be classified into 4 types, which are hydrophilic colloid, fine solid phase, surface active agent and surfactant. These agents have both a hydrophilic and a lipophilic part in their chemical structure. All emulsifying agents concentrate at and are adsorbed onto the oil:water interface to provide a protective barrier around the dispersed droplets. In addition to this protective barrier, emulsifiers stabilize the emulsion by reducing the interfacial tension of the system. Some agents enhance stability by imparting a charge on the droplet surface thus reducing the physical contact between the droplets and decreasing the potential for coalescence. Some commonly used emulsifying agents include tragacanth, sodium lauryl sulfate, sodium dioctyl sulfosuccinate, and polymers known as the Spans and Tweens. The HLB (hydrophilic-lipophilic balance) have been used to determine the quantity and the type of surfactant need to be used to prepare a stable emulsion. Every surfactant has it own HLB range which is from 1 (lipophilic) until 20 (hydrophilic). Normally, the usage of 2 emulsifying agent will form a very stabilize emulsion preparation. The HLB value can be determine using the equation below:  Apparatus:
Apparatus:
8 tests tube
50ml measuring cylinder
2 sets pasture pipette and dropper
Vortex mixture
Weighing boats
1 set mortar & pestle
Light Microscope
Microscope slides
A set of 5ml pipette and bulb
A 50mL beaker
A 15ml centrifugation tube
Coulter counter apparatus
Centrifugation apparatus
Viscometer
Water bath 45oC
Refrigerator 4°
Reagents:
Mineral oil
Arachis oil
Olive oil
Distilled water
Span 20
Tween 80
Sudan III solution
Procedures:
1. 8 tests tube is labeled and 1cm from the bottom is marked at the tests tube.
2. 4mL of oil (refer to table I) and 4ml of distilled water is mixed in the test tube. 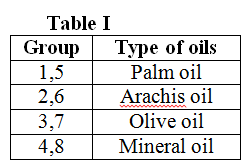 3. Span 20 and Tween 80 is added to the mixture according to the amount given in the table below. The mixture is mixed using the Vortex mixing machine for about 45 seconds. The time taken for separation to occur until it reaches the 1cm marked is recorded. The HLB value for each sample is determined.
3. Span 20 and Tween 80 is added to the mixture according to the amount given in the table below. The mixture is mixed using the Vortex mixing machine for about 45 seconds. The time taken for separation to occur until it reaches the 1cm marked is recorded. The HLB value for each sample is determined.  4. The Sudan III mixture is dropped into 1g of each of the emulsion produced in the weighing boats. The color dispersion is described and compared with other emulsion formulation. The emulsion is observed under the light microscope. The structure and globule size are determined and drawn to compare with other emulsion.
4. The Sudan III mixture is dropped into 1g of each of the emulsion produced in the weighing boats. The color dispersion is described and compared with other emulsion formulation. The emulsion is observed under the light microscope. The structure and globule size are determined and drawn to compare with other emulsion.
5. The Mineral Oil Emulsion (50g) is prepared using wet gum method following the formulation below:  6. 40g of the emulsion is placed in a 50ml beaker and homogenize for 2 minute using homogenizer.
6. 40g of the emulsion is placed in a 50ml beaker and homogenize for 2 minute using homogenizer.
7. 2g of the sample before and after been homogenized is taken out and placed in the weighing boats. Sudan III solution is dropped into the emulsion. The texture, consistency, appearance of the oil and the color dispersion is determined and compared which is it is observed under the light microscope.
8. 15g of the emulsion that have been homogenized is taken and the viscosity is determined using the viscometer that has been calibrated using the “Spindle” LV-4 type. The sample is placed at 45°C for about 30 minutes and at 4°C for 30 minutes afterward. The viscosity is determined afterward.
9. 5g of the emulsion is centrifuged in 4500rpm, for 30 minutes at 25oC. The separation is measured and the ratio is determined.
Results and Calculation:
Phase separation
The stability is indicated by the symbol below:  Calculation of HLB values:
Calculation of HLB values: ![]()

 Table II
Table II 




 Viscosity of emulsion:
Viscosity of emulsion: 


 Separation height
Separation height 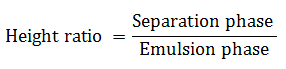
20mL mineral oil
25mL mineral oil
30mL mineral oil
35mL mineral oil

 Discussions: 1. What are the HLB values needed to produce a stable emulsion? Discuss.
Discussions: 1. What are the HLB values needed to produce a stable emulsion? Discuss.  A system was developed to assist in making systemic decisions about the amounts and types of surfactants needed in stable products and the system is called the HLB (hydrophile-lipophile balance) system and has an arbitrary scale of 1 – 18. HLB numbers are experimentally determined for the different emulsifiers. If an emulsifier has a low HLB number, there is a low number of hydrophilic groups on the molecule and it will have more of a lipophilic character. For example, the Spans® generally have low HLB numbers and they are also oil soluble. Because of their oil soluble character, Spans® will cause the oil phase to predominate and form an w/o emulsion.
A system was developed to assist in making systemic decisions about the amounts and types of surfactants needed in stable products and the system is called the HLB (hydrophile-lipophile balance) system and has an arbitrary scale of 1 – 18. HLB numbers are experimentally determined for the different emulsifiers. If an emulsifier has a low HLB number, there is a low number of hydrophilic groups on the molecule and it will have more of a lipophilic character. For example, the Spans® generally have low HLB numbers and they are also oil soluble. Because of their oil soluble character, Spans® will cause the oil phase to predominate and form an w/o emulsion.
The higher HLB number would indicate that the emulsifier has a large number of hydrophilic groups on the molecule and therefore should be more hydrophilic in character. The Tweens® have higher HLB numbers and they are also water soluble.
Because of their water soluble character, Tweens® will cause the water phase to predominate and form an o/w emulsion. Combinations of emulsifiers can produce more stable emulsions than using a single emulsifier with the same HLB number. Surfactant possesses approximately an equal ratio between the polar and non-polar portions of each molecule.
When placed in an oil-water system, the polar groups are attracted to or orient toward the water, and the non-polar groups are oriented toward the oil. The surfactant molecule lowers the interfacial tension between the oil and water phases. Surfactants are classified as cationic, anionic and nonionic based on the type of polar group on the surfactant. Cationic surfactants are often used as antibacterial agents because of their ability to disrupt the cell membrane of the microorganism.
The ionized surfactants have a relatively high water solubility and thus generally make oil in water emulsions. The nonionic surfactants, however, can be used to make either type of emulsion. Different types of oil used to prepare an emulsion will need different optimum HLB values. In this experiment, the optimum HLB values for olive oil to produce the most stable emulsion is 11.37 where it produces longer time for phase separation to occur which is 60 minutes.
This is because the slower the phase separation time, the higher the stability of emulsion. In addition, tube 8 gives the lowest stability of emulsion produced with HLB value of 0. This is because there is no use of surfactant (Span 20 or Tween 80) in this tube to aid the dispersion of oil phase into aqueous phase (oil in water emulsion) or dispersion of aqueous phase into oil phase (water in oil emulsion) through the formation of micelles. Hence, phase separation occurs in the shortest time in Tube 8 of 15 minutes.
2. Compare the physical features of mineral oil emulsion and give explanation. What is Sudan III Test? Compare dispersion of color in the emulsion formed and give explanation.
Sudan III is predominantly used for staining triglycerides in animal tissues (frozen sections). Sudan dyes are a group of lipid soluble solvent dyes often called lysochromes, in structural classification they are diazo dyes. With the use of certain solvents, may also be used to stain some protein bound lipids in paraffin sections.
Sudan I, Sudan III and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan III solution has 86% dye dissolved in a volume of ethyl alcohol about 95% v/v. Sudan III is fat soluble dye which can be used for staining oil. It is red in colour and will dissolve in the oil phase to give a red colour to the oily globules. The aqueous phase globules will remain colourless.
Hence, in this experiment, Sudan III is used to determine the type of emulsion formed. We can classify the emulsion into oil-in-water emulsion (o/w emulsion) or water-in-oil (w/o emulsion) when the colour dispersion is viewed under the microscope. 



 From this experiment, there’s an obvious change on emulsion before and after homogenization. Before homogenization, the emulsion is not broken down into smaller globules. Therefore, the emulsifier, acacia is not well distributed around the globules in the emulsion. Unstable and large globules are formed and tend to coalesce with each other. This emulsion is unstable and have high tendency for destabilization process to occur. After homogenization, the globules are broken down into smaller one. The oil and water are dispersed evenly.
From this experiment, there’s an obvious change on emulsion before and after homogenization. Before homogenization, the emulsion is not broken down into smaller globules. Therefore, the emulsifier, acacia is not well distributed around the globules in the emulsion. Unstable and large globules are formed and tend to coalesce with each other. This emulsion is unstable and have high tendency for destabilization process to occur. After homogenization, the globules are broken down into smaller one. The oil and water are dispersed evenly.
Hence, the colour become whiter compared to emulsion before homogenization. The emulsifier may evenly adsorb on the interfacial surface of globules and promote stable emulsion. This process also helps to break down the clumps of vanillin crystals and distribute evenly in the emulsion. It can be concluded that the homogenization process makes the oily globules more stable in the aqueous phase.
3. Plot and give comments on: a) Graph of the sample’s viscosity before and after the temperature cycle against the variety contents of mineral oil. 
 Acacia, which is hydrocolloid emulsifier changed by increasing the drop volume fraction, which leads to an increase of the overall emulsion viscosity. Before undergoing temperature cycle, the viscosity of the emulsion at room temperature increased when the content of the mineral oil increased from 20ml to 30ml. The viscosity has also increased after the emulsion undergo the temperature cycle.
Acacia, which is hydrocolloid emulsifier changed by increasing the drop volume fraction, which leads to an increase of the overall emulsion viscosity. Before undergoing temperature cycle, the viscosity of the emulsion at room temperature increased when the content of the mineral oil increased from 20ml to 30ml. The viscosity has also increased after the emulsion undergo the temperature cycle.
Therefore, the viscosity of concentrated emulsions increases with the increases in the volume fraction of oil, which is the dispersed phase and the increase in viscosity ratio of the dispersed phase to the continuous phase. However, there is decrease in the viscosity from 30ml to 35ml. This is due to change in oil-water ratio that can lead to change in viscosity. Greater amounts of oil in emulsion will decrease the viscosity. In addition, the HLB of nonionic emulsifier changes with temperature. The higher the temperature, the lower the HLB value of acacia. Thus, it will become hydrophobic. At temperature at which the emulsifier has equal hydrophobic and hydrophilic tendencies, phase inversion occurs. The higher viscosity of the emulsion after temperature cycle shows that the emulsion formed becoming water in oil emulsions which generally have the higher viscosity compared to oil in water emulsions.
The emulsion sample is put in the water bath at 450C for 30 minutes in the temperature cycle. Emulsion viscosity decreases with increasing temperature as increase in thermal energy of droplets and the emulsifying agent at oil in water interface, therefore, increases the frequency of droplets collisions, reduces the interfacial viscosity, which results in faster drop coalescence. Solubility of emulsifiers increases when temperature increases as increase the ability to leave its own and break out of the emulsion.
Emulsion becomes unstable, breaks apart and viscosity decreases. After that, it is put into freezer at 40C for 30 minutes. Viscosity of emulsion increases when it is cooled as kinetic energy of droplets of the system is reduced. Thus, this will decrease the rate of migration of the globules in the disperse phase. Therefore, there is increase in viscosity in emulsion after temperature cycle in mineral oil emulsion.
b) Graph of the difference of viscosity (%) against the different oil contents  Viscosity of emulsion after temperature cycle will be higher than viscosity of emulsion before temperature cycle due to the phase inversion has occurred when there is change in temperature. Therefore, there are positive value results shown in viscosity difference in different volume of mineral oil. The smaller the amount of mineral oil used, the higher the increase in percentage of viscosity in emulsion. Thus, the larger the difference in viscosity, the more instable the emulsion will be.
Viscosity of emulsion after temperature cycle will be higher than viscosity of emulsion before temperature cycle due to the phase inversion has occurred when there is change in temperature. Therefore, there are positive value results shown in viscosity difference in different volume of mineral oil. The smaller the amount of mineral oil used, the higher the increase in percentage of viscosity in emulsion. Thus, the larger the difference in viscosity, the more instable the emulsion will be.
There is increase in viscosity in increasing amount of oil. Therefore, small viscosity change should be shown as the less effort is needed to change the viscosity as it already possesses the highest viscosity in increasing amount of oil in emulsion. In experiment, 35ml mineral oil in emulsion has the lowest viscosity difference as it contains larger amount of oil. However, there is some deviation for 25ml and 30ml which does not follow the trend. This is because readings that are taken between two groups show an extreme value. This may be due to homogenization error.
4. Plot a graph of ratio of phase separation against different amount of mineral oil. Give comments.  Based on graph at the above,the ratio of phase separation increases as the content of mineral oil increases. The higher the content of mineral oil used, the higher will be the separated phase ratio (separate into two non-homogenous phases), and the emulsion formed will be unstable. In order to produce a stable and homogenous emulsion, the phase separation ratio must always be kept at minimum level so that the drug can be dispersed uniformly in the emulsion and the administration of accurate dose can be achieved.
Based on graph at the above,the ratio of phase separation increases as the content of mineral oil increases. The higher the content of mineral oil used, the higher will be the separated phase ratio (separate into two non-homogenous phases), and the emulsion formed will be unstable. In order to produce a stable and homogenous emulsion, the phase separation ratio must always be kept at minimum level so that the drug can be dispersed uniformly in the emulsion and the administration of accurate dose can be achieved.
An error may occurred during the experiment if the reduction of phase separation ratio occured. For example, if the chemicals or materials used are already contaminated, the data obtained will become inaccurate.
5. What are the functions of every substance used in this emulsion preparation? How the different contents of substances affect the physical characteristics and stability in the formulation of an emulsion?
Mineral oil used in this emulsion preparation as the oil phase. It can be either continuous phase or dispersed phase based on its amount. The stable range for disperse phase is 30-60%. If the disperse phase approaches or exceed 74% of the formulation it will become unstable. Spans and Tweens are complex esters, derived from polyols, alkylene oxides, fatty acids, and fatty alcohols.
They are used frequently in compounding because they are both stable to heat and are also stable over a wide range of pH values. Acacia is naturally occurring polysaccharides serves as emulsifying agent. Acacia acts as a ‘gum’ to bind the water and oil molecule together so that they can mix.
Acacia will form a thick film at oil-water interface to act as barrier to coalescence. Acacia also function as stabilizer, adhesive, flavor fixative and inhibitor of sugar crystallization. Syrup acts as sweetening agent to mask the non-palatable taste of the mineral oil in order to increase patients’ compliance. Syrup may also acts viscosity-enhancing agents to increase the viscosity of the emulsion and ease of pour ability. Alcohol is added as preservatives to inhibit the growth of microorganisms because of the presence of water, syrup and acacia which may enhance the development of microorganism in the oil in this emulsion.
Vanillin functioned as flavouring agent to improve the taste so that it will be palatable and be more accepted by patients. In this experiment, the different composition of mineral oil used affects the physical properties and stability of the emulsion. The stable range for disperse phase is 30-60%. If the disperse phase approaches or exceeds 74% phase inversion may occur. Oil in water emulsion will be produced if the amount of distilled water used is in excess compared to the oil used while water in oil emulsion is produced if the amount of oil used is in excess.
Viscosity will increase in addition of more dispersed phase up to a certain point. But decrease in stability after that point. Phase inversion may also occur with addition of substances that can alter the solubility of emulsifying agent. The most stable emulsion is when the contact angle of immersed particles is 90◦.
Conclusion:
Low HLB surfactant value, 3-9 is suitable to be used in water in oil emulsion. While, the high value of HLB surfactant, 8-18 is suitable for the formation of oil in water emulsion. Combination of surfactants, such as Span and Tween will form a more stable emulsion than a single surfactant. Different oily phase need a different value of HLB surfactant so that the most stable emulsion can be formed.
References :
1. Pharmaceutical Practice, A.J. Winfield, J.A. Rees, I.Smith, 4th edition
2. Composition And Properties Of Drilling And Completion Fluid, R.Caenn, G.R. Grey, H.C.H. Darley, 6th edition



